True Carbon Neutrality in Glass
Some challenges to reach net zero CO2 emissions by 2050
By Chris Hoyle, Sr. Vice President-Technical Director; Brian J. Naveken, Mgr. of Technology & Technical Sales
Paths to Carbon Neutrality
There is an English expression known as “the elephant in the room” which refers to an important or enormous topic that is obvious, where no one mentions or discusses it but needs to be addressed. In the case of carbon neutrality in glass, it is the economic considerations.
There are basically three paths to carbon neutrality for glass manufacturing. These being:
- Carbon Capture/Sequestration
- Alternative Fuels - Hydrogen, Biogas, Blends, Electricity
- Batch Modifications – Increased cullet and elimination of carbonate materials
The paths are not mutually exclusive, they can be combined to increase the reduction in CO2 emissions
Interestingly, the second item, alternative fuels, does not address CO2 emissions from the batch. So, carbon capture would still be required and/or the use of carbon credits. In this article, the economics, more specifically the operating expense (OPEX) considerations are going to be limited to alternate fuels, specifically hydrogen (H2) and batch materials.
Batch Materials

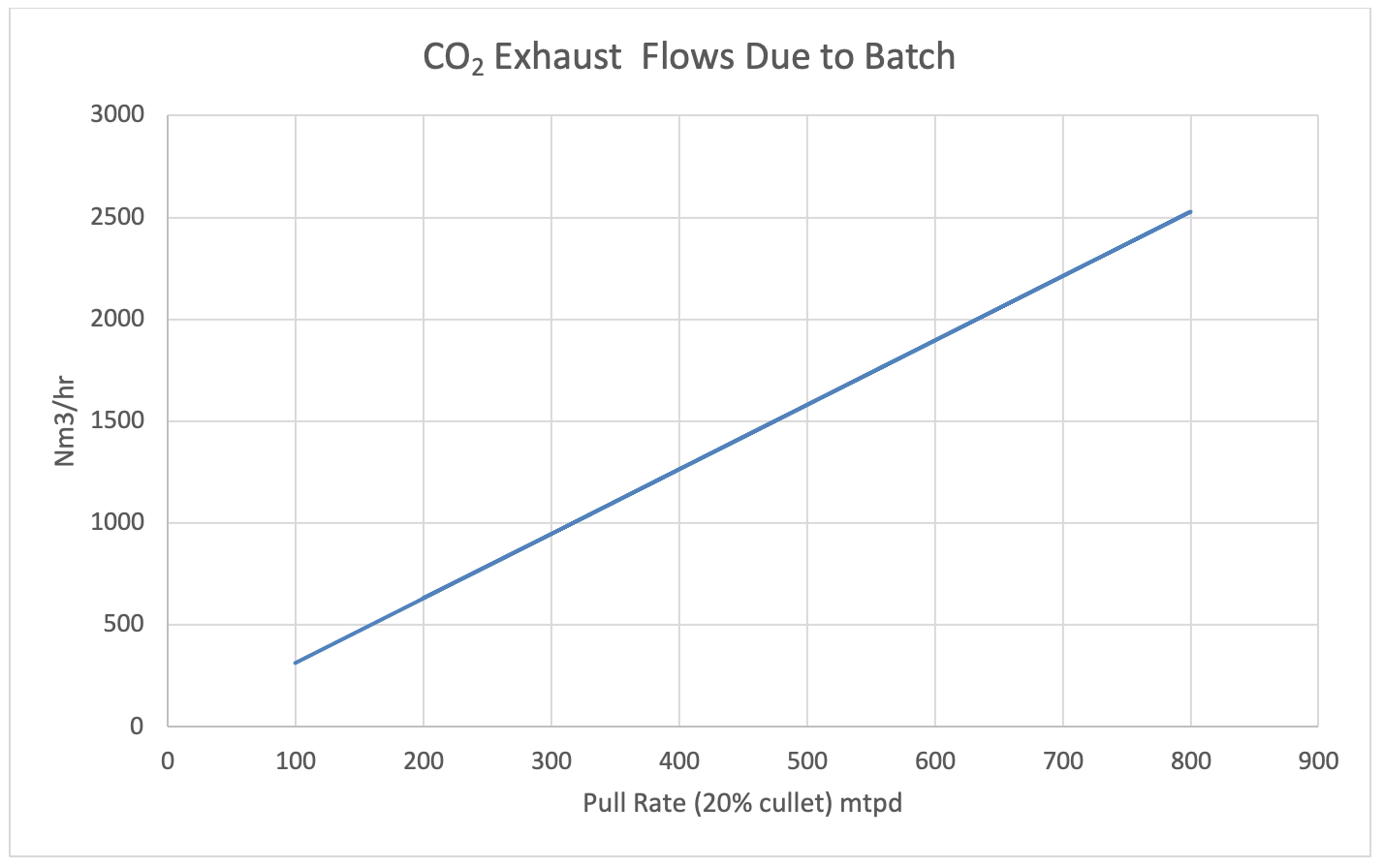
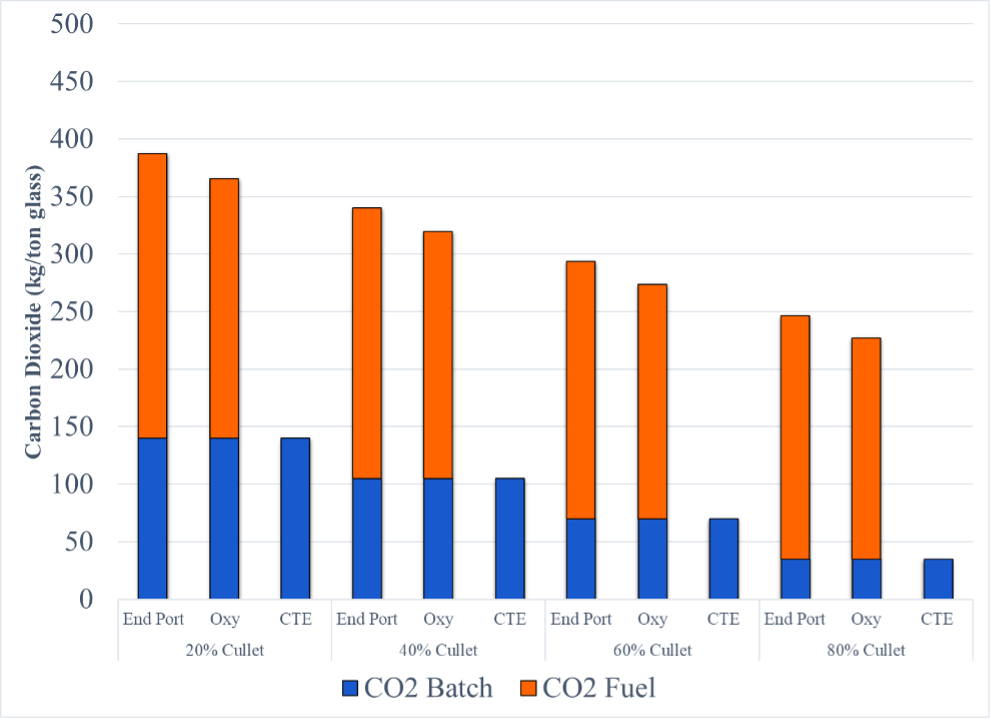
At 20% cullet approximately 35% of CO2 emissions from the production of glass is from the melting of the batch materials and has not received the attention that emissions from fuel has gotten. Other CO2 emissions by cullet percentages are represented in Figure 3.
In a basic equation for the production of glass,
SiO2 + Na2O + CaO → Soda Lime Silicate Glass
there contains no carbon dioxide (CO2) product formation. The formation of CO2 is a product of using the carbonate forms of the Na2O and the CaO more commonly known as soda ash (Na2CO3) and limestone (CaCO3). The resulting equation now becomes,
SiO2 + Na2CO3 + CaCO3 → Soda Lime Silicate Glass + CO2
Figure 2 shows some typical volumetric (normal) and mass flows of exhaust CO2 due to batch.
There are two main reasons why the carbonate form of the raw materials is used. These being the abundance of the raw materials, which also results in a lower cost, and secondly the melting of batch takes less energy. By using soda ash and limestone, this reduces their respective batch material cost by approximately 4-5 and 1.5-2 times. Alternatively, batch materials from recycled sources, such as water treatment waste and fly ash, can be used but this will only reduce the carbon footprint by 4-10%. To get to complete neutrality, carbon capture/sequestration would still be needed.
Alternative Fuels
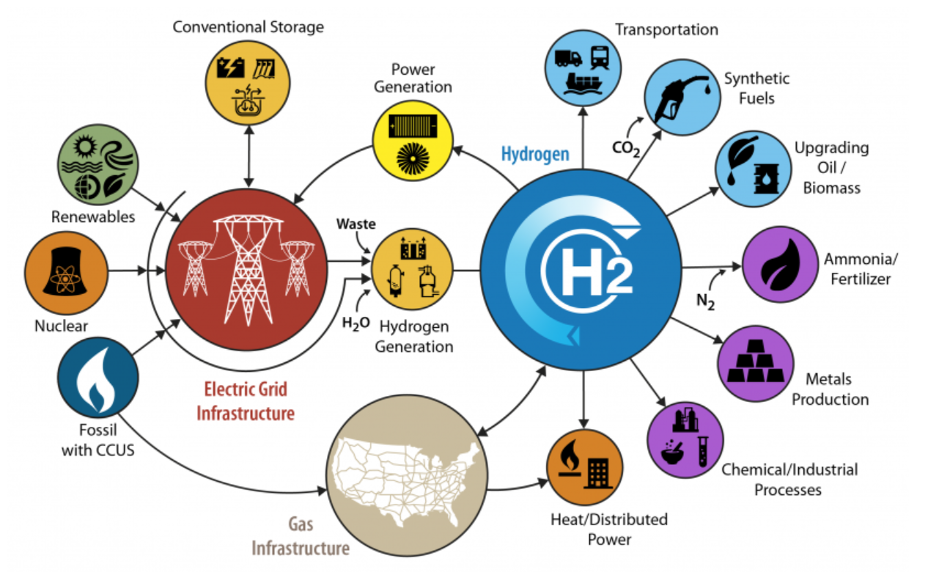
When looking at carbon neutrality, hydrogen (H2) looks to be the most viable candidate, and this can be seen in the research and the billions of dollars in potential funding for hydrogen hubs and infrastructures. In the United States alone there is a Department of Energy funding opportunity of $8 billion for the five (5) year period encompassing fiscal years 2022 through 2026 for the development of regional clean hydrogen hubs that demonstrate the production, processing, delivery, storage, and end-use of clean hydrogen. This concept is presented in Figure 4.
The basic equation for the combustion of natural gas using air or oxygen is,
CxHy + zO2 → xCO2 + (y/2) H2O
Natural gas is primarily made up of methane which combusts as,
CH4 + 2O2 → CO2 + 2H2O
Now, if we look at hydrogen, the CO2 product gets eliminated.
2H2 + O2 → 2H2O
Perfect! The H2 combustion eliminates of 100% of the CO2 from the gaseous energy source for melting and refining glass (note: all electric melting does the same). But let’s look a bit closer. The heating value of natural gas is ~36.73 MJ/Nm3
as compared to hydrogen’s ~10.78 MJ/Nm3. What this means to glass melting is:
- Will need ~3-3.5x the volume of hydrogen than natural gas
- Will have to account for the effects on distribution and storage of H2
- Will produce ~1.5x more water vapor in exhaust
- What effects will the water have on glass and refractories?
- Will need ~25% less oxygen (economic advantage over Oxy-Fuel furnaces)
- Exhaust mass flow and velocity negligible
- The higher flame speed of H2, higher permeability, and hydrogen embrittlement will require greater safety measures
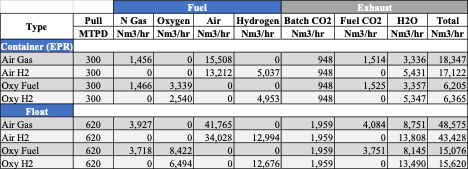
Figure 5 shows the comparison of a container and float furnace using air-gas or oxy-fuel and replacing the fuel type with hydrogen with all other inputs remaining the same.
The economic practicality is constantly changing and will continue to change due to energy prices and the processes used to generate hydrogen. Taking the first bullet point from above, in order to get the same melting energy from hydrogen you need to increase the volumetric flow of hydrogen by three to three and half times. If hydrogen cost gets comparable to natural gas on a volumetric basis, which currently it is two to three times higher, one’s energy cost would still be triple due to the increased volume of hydrogen needed. To put the volume of hydrogen into a bit more perspective, for a float furnace to convert their port 0 oxy-fuel burners to hydrogen would require five times more hydrogen than what they use to operate their tin bath. However, by 2050, the natural gas economic advantage may disappear with the addition of carbon taxes.
Technical Feasibility of Hydrogen in Glass Furnaces
The first questions for the success of hydrogen combustion is how will the hydrogen be generated, transported, stored and ultimately combusted in existing glass furnaces? Or will all new furnaces designs be needed? Or just modifications to existing furnaces?
Some of the considerations for furnace conversions from natural gas to hydrogen are:
- Mechanical Systems – The molecular weight of hydrogen is 2 and methane is 16 which means hydrogen is a smaller molecule and considerations for materials to reduce leaks at valves, gaskets, etc. need to be met.
- Effects on refractory due to increased water in the exhaust
- Flame control and speed
- Heat transmittance – over the batch blanket as well as glass due to the lack of carbon in the fuel which lowers the flame luminosity and affects radiant heat transfer
Side Note – Electrical Furnaces
With all electric furnaces CO2 emissions are eliminated to same degree as hydrogen combustion and has been a production proven and economical method of melting glass. The biggest drawback is glass quality but with the same degree of effort and innovation that hydrogen combustion is receiving, the fining/refining issues should be able to be overcome.
Conclusion
Carbon emissions from batch materials is significant. At 20% cullet it accounts for approximately 35% of the CO2. Non-carbonate forms of soda ash and limestone will need to addressed while considering economic and supply considerations. Using hydrogen as an alternative, the price of hydrogen needs to be reduced considerably in relation to natural gas.
All electric furnaces from a fuel standpoint produce no CO2 like H2 and have been production proven for many years by the leaders in all electric melting, The TECO Group. More innovation into the refining and fining of glass using all electric may ultimately make the most economic sense.
Internationally there is a considerable decarbonization effort going on within the glass community as well as other industries. Hydrogen trials in glass furnaces have begun and will continue. Refractory companies are conducting tests for increased water content in the exhaust and burner companies are developing new designs for hydrogen combustion. The challenges to decarbonize glass production are numerous and demand innovation along multiple paths, from raw materials to adaptation of hydrogen combustion, to advances in all electric melting. The TECO Group and their seven (7) companies are uniquely positioned to solve clients’ issues, in this case decarbonization, by delivering unique solutions that creates value through innovation within a framework to manage risk.






Add a Comment